
MENUMENU
TALK TO AN EXPERT
Special Hours: 7AM – 6PM PST
TALK TO AN EXPERT
Special Hours: 7AM – 6PM PST
If you were today-years-old when you learned what an anode vs cathode was, you’re not alone. Most of us rarely deal with these terms unless we’re fixing a water heater or installing your own vehicle or boat batteries. So, if you’re looking for an article that spells out the difference in plain English, look no further. Here we discuss what an anode is, what a cathode is, how they both work, and where they’re used.
Let’s get started!
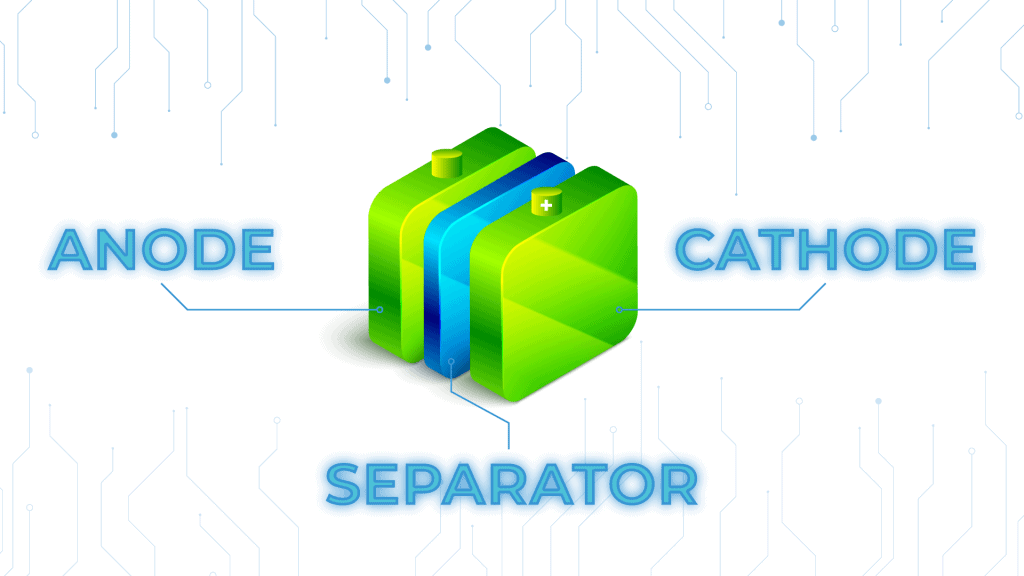
Trying to understand how batteries work can feel like learning another language, especially if you barely remember high school chemistry. Below, we dissect the components a battery will need to charge and emit power (in a bite-sized, understandable way).
An anode is a negative electrode and it’s one of the essential parts of a battery. It’s usually made of a metal that oxidizes and sends electrons to the cathode (the positive electrode). This is an electrochemical reaction that produces electrons (i.e., electricity).
An anode is an oxidizing metal, such as zinc or lithium, which means it loses electrons. It resides in an electrolyte solvent and slowly erodes as electrons move along a conductor to the cathode.
The conductor (whether it be a metal wire or tube) is how we access the electricity the anode makes, and ultimately, how a battery powers our devices. Once the anode completely erodes, the battery dies (or loses charge).
Anodes can come from several different materials. These include zinc, lithium, graphite, or platinum. A good anode should be an efficient reducing agent, have good conductivity, stability, and a high coulombic output (the electrical energy output).
Like an anode, a cathode is one of the electrodes in a battery as well. However, a cathode is referred to as the positive electrode because it gains electrons rather than loses them. Therefore, anodes oxidize (lose electrons) while cathodes reduce (gain electrons).
Essentially, a cathode is there to receive electrons from the anode. Both the anode and cathode are submerged in an electrolyte solution, and electricity travels through the conductor from the negative to the positive parts of your battery. This, in a nutshell, is how a battery generates electricity.
To visualize how the cathode works, click here to watch a brief but fantastic video that explains this process.
A cathode can be any material as long as it’s an efficient oxidizing agent that’s stable when in contact with an electrolyte. Metallic oxides make excellent cathode materials because they have a useful working voltage as well. These include copper oxide, lithium oxide, and graphic oxide.

Anode vs cathode. In other words: How do you tell them apart on your battery?
It’s actually very simple. Most RV, car, and even household batteries have a plus (+) and minus (-) sign on each end. Because the anode is the negative electrode (and thus loses electrons), the minus sign refers to the anode. On the other hand, the plus sign refers to the cathode because it’s the positive electrode (and thus gains electrons).
It’s important to understand the difference between the anode vs cathode because you can comprehend precisely how your batteries work, whether you’re on a boat, driving a recreational vehicle, or even just changing the batteries in your remote. Whether you’re doing your own solar setup or replacing your batteries, you’ll feel confident in your abilities to properly install your device’s powerhouse.
It’s also helpful when you’re jump-starting a car. Do you ever draw a blank trying to figure out where you need to attach the booster cable clamps? Now you know–one goes on the negative end (the anode), and the other goes on the positive end (the cathode).

Plus, you can sound smart talking to your friends!
Anodes and cathodes play a role in other places besides batteries. For example, ships have “sacrificial anodes” that act as a protectant to the cathode, which is the parent material that you need to protect from corrosion.
You’ll also find anodes in household utilities. Water heaters have sacrificial anode rods that extend the life of the water heater. Essentially, the anode rod attracts minerals found in the water and erodes instead of the tank itself. Hence, the name “sacrificial.”
Anodes can also help protect fluid tanks and pipes from corrosion–always to protect the cathode (i.e. the important material that manufacturers want to preserve).

Most of us have no idea what an anode or cathode is, simply because we don’t deal with these terms in day-to-day life. However, if you own a car, recreational vehicle, boat, like to tinker, or just want to know how things work, it’s helpful to familiarize yourself with anodes vs cathodes.
After all, they’re in your batteries, water heater, and many other places in your daily life!
Have any questions about anodes and cathodes? Leave them in the comments below.
The post Anode vs Cathode: What’s the Difference? appeared first on Dragonfly Energy.
Shop Best Sellers








Ask a technical specialist now at 855.292.2831
Stay in the Know