
MENUMENU
TALK TO AN EXPERT
Special Hours: 7AM – 6PM PST
TALK TO AN EXPERT
Special Hours: 7AM – 6PM PST
There are few worse feelings than trying to start up a car, RV, boat, or other system and finding the battery completely dead. You may end up stranded and then stuck with a hefty bill for a new battery to boot. Unfortunately, it’s a relatively common occurrence for people unfamiliar with the main causes of lead-acid battery damage. So read on as we take a closer look at the lead-acid battery, how it works, and some things to avoid to keep them running.
Lead-acid batteries are a common type of rechargeable battery invented more than 160 years ago. At their core, their construction is pretty simple: Two lead plates (one positively charged, one negatively charged) suspended in an acid electrolyte solution. When a current is applied to the system, chemical reactions change the relationship of the electrolytes to the plates, charging the battery.
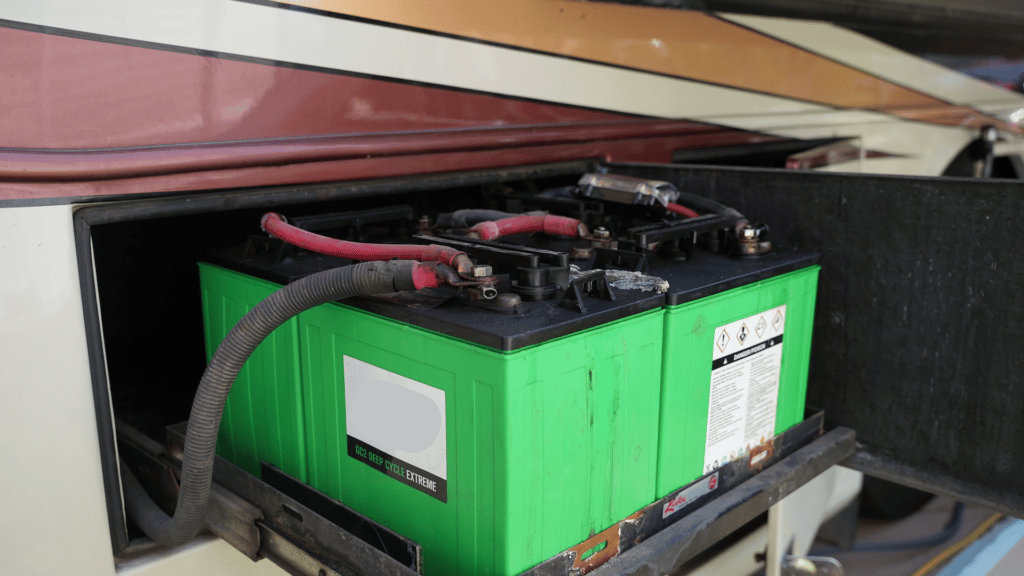
When you use your battery, the process happens in reverse, as the opposite chemical reaction generates the batteries’ electricity. In unsealed lead acid batteries, periodically, you’ll have to open up the battery and top it off with distilled water to ensure the electrolyte solution remains at the proper concentration.
Beyond this simple construction, there are a few different battery designs like AGM (absorbent glass mat) or gel batteries. Using the same basic principle with differences in construction and composition, these batteries offer slightly improved performance in some areas and fewer maintenance requirements than traditional flooded lead-acid alternatives.
Unfortunately, many things can cause lead-acid battery damage. Because these batteries run on chemical reactions, when conditions are not right for the reaction to occur, the batteries can become permanently damaged.
For example, discharging lead-acid batteries below 50% charge will increase a chemical reaction called sulfation and damage the battery. Because of this, the battery really should never put out more than half of its rated capacity, or life will be reduced.
On the flip side, charging batteries too quickly can also damage them. This causes an imbalance in the plate’s chemical changes that can impact its performance.
In addition, lead-acid must be recharged fully after every discharge cycle. If they are not recharged all the way, something called sulfation occurs that cannot be reversed. Sulfation is a crystalized lead sulfate (a combination of lead and acid in the electrolyte) that impacts the chemical reaction of the plates and electrolyte.
Additionally, operating lead-acid batteries with an electrolyte solution that’s outside the acceptable range (either too concentrated or too dilute) can also cause significant issues for the battery.
Lastly, lead acid batteries can suffer from something called stratification. This is a difference in chemical makeup in the top and bottom half of the battery due to a density difference in the chemical properties of the reagents. This is problematic if the batteries are not stirred or moved, most commonly in stationary applications.
Lastly, high temperatures can significantly damage a lead-acid battery. Any temperature above 80 degrees significantly increases the degradation of the chemicals in a battery. This causes rapid self-discharge and sulfation.
The most common mistake owners make is using lead acid in applications they are not well suited for. The only applications that a lead acid battery is operated for longevity are when they are discharged for short periods (less than 50 percent) and then fully recharged. One application that fits this need is vehicle starting.
Applications for stationary storage can have stratification and sulfation problems. Deep discharges or inconsistent recharging also is not a good fit for lead acid. Applications that have these profiles are solar energy storage and energy storage for off-grid power.
Two of the most common mistakes that lead to lead-acid battery damage involve charging — or lack thereof. Some owners discharge their batteries too deeply, permanently altering their chemistry and function. Others overcharge their batteries or charge them too quickly, which can do equal amounts of damage.
Operating in extremely hot or cold temperatures risks harming the health of your battery as well. A lack of maintenance or improper maintenance is also one of the biggest causes of damage to lead-acid batteries, generally from the electrolyte solution having too much or too little water.
All of the ways lead acid can be damaged are not issues for lithium and why our batteries are far superior for energy storage applications. Lead acid is fantastic as a starting battery, but will fail terribly in a storage application.
→ Did you know? Battle Born Lithium Batteries require virtually no maintenance and their BMS works around the clock to make sure it avoids damage from charging, discharging, and environmental factors.
One of the key ways that lead-acid battery damage reveals itself is through poor performance. Is your battery not providing the juice you need in terms of voltage or total capacity? This should lead you to investigate further.
Some damage is also plainly visible. Are there any unusual bulges, distortions, or cracks on your battery? Check for any evidence of leakage or other unexpected discharge.
→ Warning: Battery acid can be extremely corrosive and dangerous. Be sure to wear proper personal protective equipment (PPE) when working with damaged lead-acid batteries.

Overheating is always a potential risk for lead-acid batteries, especially in hot conditions or with an otherwise failing battery. While all batteries will get warm during use, lead-acid batteries that overheat can become seriously damaged. Once the electrolyte solution inside the battery reaches the boiling point, it begins to release as an acid or hydrogen gas. These vapors can be harmful if inhaled by humans.
Meanwhile, the fluid levels in your battery will continue to fall, causing damage if they drop too low. Many batteries can be topped off with more distilled water once they cool down to restore them to normal functioning. However, you’ll need to replace sealed batteries like AGM and gel styles if they overheat.
Most of the time, a lead-acid battery is simply dead. Ones that have suffered severe lead-acid battery damage or have reached the end of their average lifespan should simply be replaced.
But in other cases, it’s entirely possible to revive a lead-acid battery. If a battery seems nearly flat, try jump-starting it or connecting it to a trickle charger. These devices slowly provide a small amount of low-voltage power to the battery. This helps balance the charge inside the battery and may partially recover it.
Most problems, however, are with sulfation, and there is no good way to dissolve the crystals. There are chemical solutions you can add to your electrolyte mix that may dissolve sulfates, but using them is a dangerous process.
Yes, but getting an accurate reading of capacity is very hard. Testing to see if it is putting out voltage is very easy, however. All you’ll need is an ordinary digital voltmeter.
Touch the positive end of the voltmeter to the battery’s positive terminal and the negative to the negative. Within moments, you’ll see the battery’s voltage appear.
In most cases, you’ll want to see a reading above 12.4 but less than 12.9. However, some sources suggest slightly higher or lower acceptable readings. Within those boundaries, you can rest assured your battery is working correctly. Readings from a fully-charged battery below this range may indicate issues with holding a charge properly. Over 12.9 would indicate excessive voltage within the battery, a serious issue that could damage sensitive electronics connected to it.
The only way to properly test the actual capacity of the battery requires discharging it with special equipment to measure its actual capacity.
There are a few quick ways to know when to replace your battery.
First, use the voltmeter testing method discussed above. If you find a low voltage reading, try charging the battery and testing again. A consistently low voltage is a telltale sign the battery has passed its prime.
In some uses (like RV house batteries), you may notice a definite decrease in capacity, with your batteries running down faster under the same usage as always. Finally, some battery owners replace their batteries proactively, based on their expected lifespan — two to three years for deep-cycle and five to eight years for starter and car batteries.

Of course, you can avoid all of these concerns about lead-acid battery damage and other issues simply by switching to a better battery alternative. That’s what battery owners will find in lithium batteries, which have advanced in leaps and bounds in recent years.
For one, lithium batteries have a far higher capacity relative to their weight. This means you can add capacity while maintaining your weight or even cut weight (for situations like sailboats or some RVs). Lithium batteries can operate in more extreme temperatures. You can discharge them fully without damaging them, unliked old-school batteries. They can also charge significantly faster and require essentially no maintenance.
While some may worry about the higher price of lithium compared to lead acid, you shouldn’t be. While it’s true that you’ll need to pay a bit more upfront for lithium, they’ll have a much longer lifespan than traditional batteries — two to three times as long! Over the long run, many lithium owners find they spend less than they would have to buy multiple, cheaper lead-acid batteries.
There are some situations where a lead-acid battery is a perfectly suitable solution. This is especially true for starting battery needs or ones where battery performance isn’t the top priority. In starting applications, the battery is discharged quickly, then immediately recharged by the engine. The movement of the vehicle also prevents stratification.
However, for many users, it’s simply not worth the many hassles and drawbacks of these old-school batteries. Superior lithium technology continues to improve, offering numerous benefits, from more efficient performance to less maintenance.
While everyone may not be ready to upgrade yet, the trend is clear. #LeadisDead and lithium-ion batteries will continue to expand their presence in the battery world.
We know that building or upgrading an electrical system can be overwhelming, so we’re here to help. Our Reno, Nevada-based sales and customer service team is standing by at (855) 292-2831 to take your questions!
Also, join us on Facebook, Instagram, and YouTube to learn more about how lithium battery systems can power your lifestyle, see how others have built their systems, and gain the confidence to get out there and stay out there.
Shop Best Sellers








Ask a technical specialist now at 855.292.2831
Stay in the Know
One thought on “What Will Kill My Lead-Acid Battery?”
I need to know the price of lithium-ion battery from 50Ahr to 250Ahr.
Thanks